11.12.2022
Dear aquarists,
You may still remember when we examined different reactor media in 2019 with regard to their release of barium and silicon.
We were able to show that (unlike what was generally known and claimed at the time) zeolite in particular releases significant barium into the water – and that activated carbon (alongside the source water) is the main source of silicon in the reef aquarium.
But what other elements are released? And what elements are adsorbed from the water column by these reactor media? Time to devote ourselves to these questions. With the seawater ICP-MS analysis established by Oceamo, we can reliably determine trace elements and pollutants at very low concentration levels: And thus learn much more about the reactor media that are frequently used.

Disclaimer: This mini-study does not claim to be complete. The material can differ significantly depending on the manufacturer/batch and thus have different properties in adsorption and leaching – the data shown cannot therefore be applied to all adsorbers/carbons, etc. , but are intended to show which properties the respective reactor media can have. It should also be pointed out that elements can be present in water in different chemical forms and the adsorption behavior can differ as a result.
Our data show the initial adsorption. As a result, displacement reactions can occur, so that elements are displaced from the adsorber when elements bind with higher affinity.
Method: Columns were loaded with a defined amount of reactor medium and rinsed with ultrapure water followed by natural seawater. Then 10 bed volumes of sample solution (natural seawater, partly with element spikes) were slowly allowed to drip through the media bed. A 0.2 µm filter (PET membrane) served as flow restrictor and to remove particles . A sample aliquot was then collected. The sample aliquots (after passage through the media) and the native sample solution were analyzed using our Oceamo ICP-MS analysis package (which also contains anion chromatography).
Well-known iron and aluminium based phosphate adsorbers, a commonly used activated carbon and typical medium-grain zeolite material were included in the study.
But now to the data. We look at major elements, trace elements and pollutants of the reactor media one after
Major elements
The charts can have bars up and down. A bar at the top means that the respective element is released by the reactor medium into the water (the higher the bar, the more significant the release).
A bar pointing downwards indicates the percentual reduction in our sample solution after passage through the reactor medium.

The aluminium-based adsorber is generally able to bind alkaline earth metals (calcium, magnesium, strontium). The boron concentration in the sample was also reduced.

In the case of zeolithe, there are no particular abnormalities in the major elements; calcium, potassium and strontium are released in small amounts into the water.

Activated carbon also had only a minor effect on the major elements and led to a slight reduction in the boron content. Other major elements were not affected.
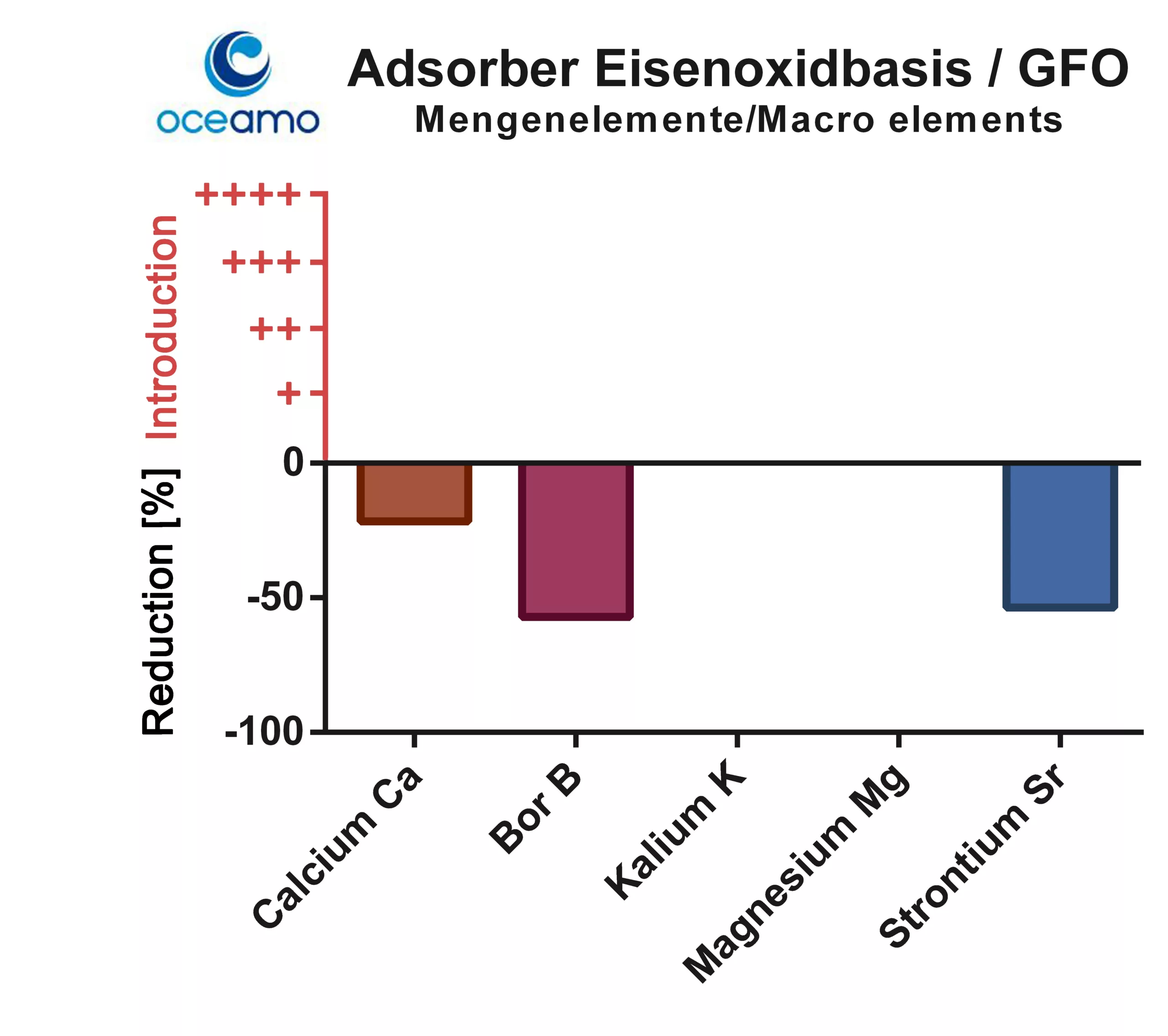
The boron concentration decreased by almost 60% compared to the starting solution. Strontium was also bound by the adsorber.
Trace elements
Especially interesting is the influence on trace elements, since excessive use of adsorbers can result in deficiency situations, which should be counteracted by increased dosing.
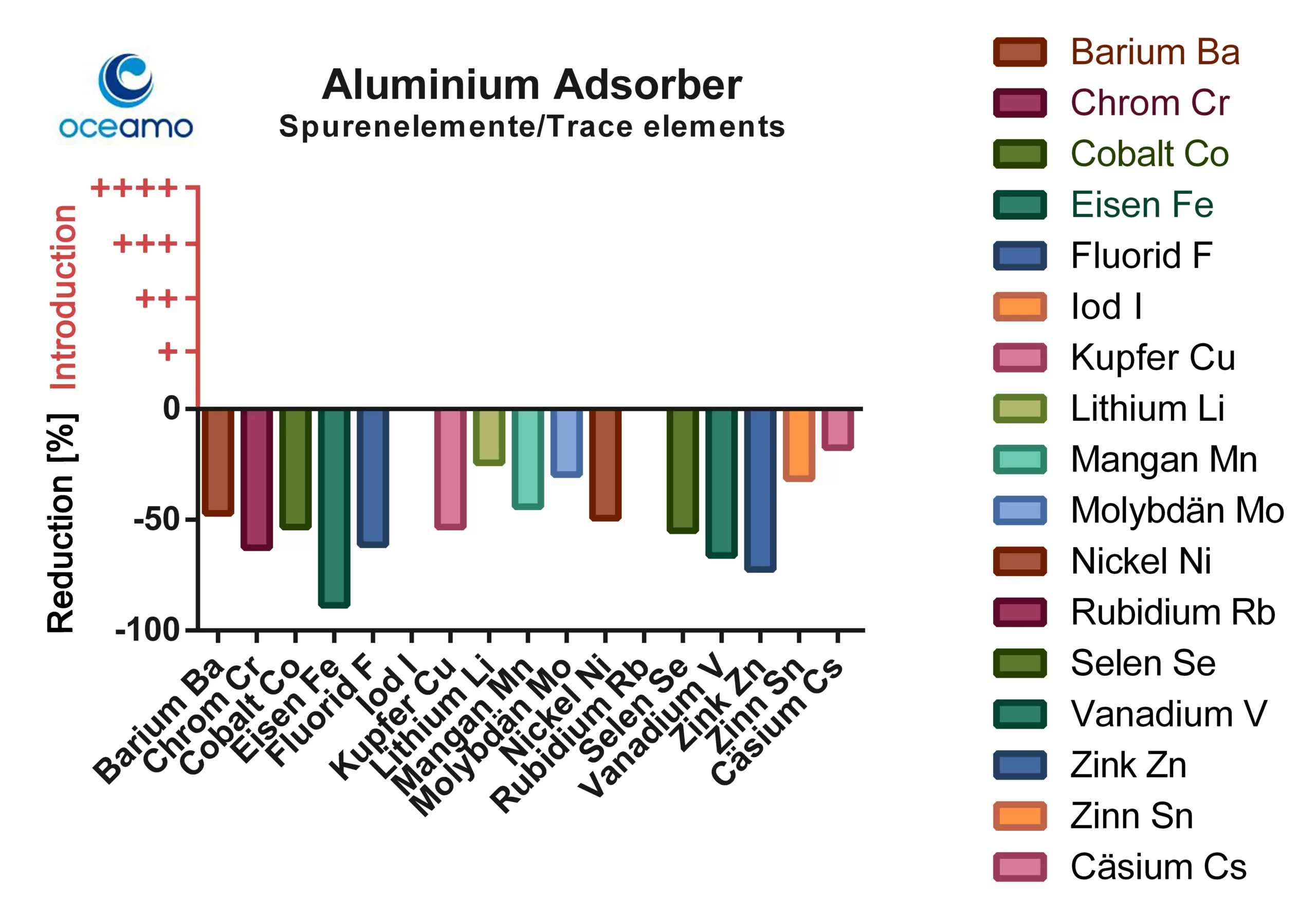
In the case of aluminium-based adsorbers, there was no release of trace elements, but a large number of essential elements were effectively bound. A significant binding of the important ultra-trace elements chromium, cobalt, iron, copper, selenium, vanadium and zinc is particularly striking.
The fluoride concentration is also significantly reduced (which is not surprising given the chemical affinity of fluoride to aluminum). The adsober had no influence on the concentration of iodine and rubidium. The influence on caesium, lithium, tin and molybdenum was also significantly less pronounced.

The examined zeolite (like other zeolites examined by us) released significant amounts of barium. Zeolites are (besides increased concentrations in some sea salts) the main reason for increased barium levels in aquariums.
Rubidium and caesium are also released into the water by typical zeolites and may contribute to the effects that zeolites have on corals. In addition, we observed a small release of manganese.
Theconcentrations of chromium and iron were significantly reduced– there was a less significant influence on fluoride, copper, vanadium, zinc and tin.
The zeolite had no effect on the concentration of other trace elements (cobalt, iodine, lithium, molybdenum, nickel, selenium).

With regard to trace elements, the activated carbon examined by us leads to an introduction of barium, cobalt, manganese, molybdenum and nickel into the water column.
The concentrations of iron, copper, zinc and tin were significantly reduced. The impact on iodine (present primarily as iodide) was less than is often assumed.
Activated carbon had no effect on fluoride, lithium, rubidium, selenium, vanadium and caesium.

What was particularly astonishing for us was the strong influence of the iron-based adsorber on almost all trace elements.
Barium, chromium, cobalt, iron, fluoride, copper, manganese, molybdenum, nickel, selenium, vanadium, zinc and tin were (almost) completely removed from the sample in our laboratory test. A deficiency situation triggered by the use of adsorbers can therefore be assessed as quite probable.
The iron-based adsorber had no effect on lithium, rubidium and caesium. The influence on iodine was also only slight.
Pollutants

Not surprising, the aluminium-based adsorber leads to a significant introduction of aluminium. What surprised us was an unexpectedly small influence on the concentration of silicon.
Antimony, uranium, arsenic and tellurium were also bound to a small extent – the adsorber had no effect on the cadmium concentration.
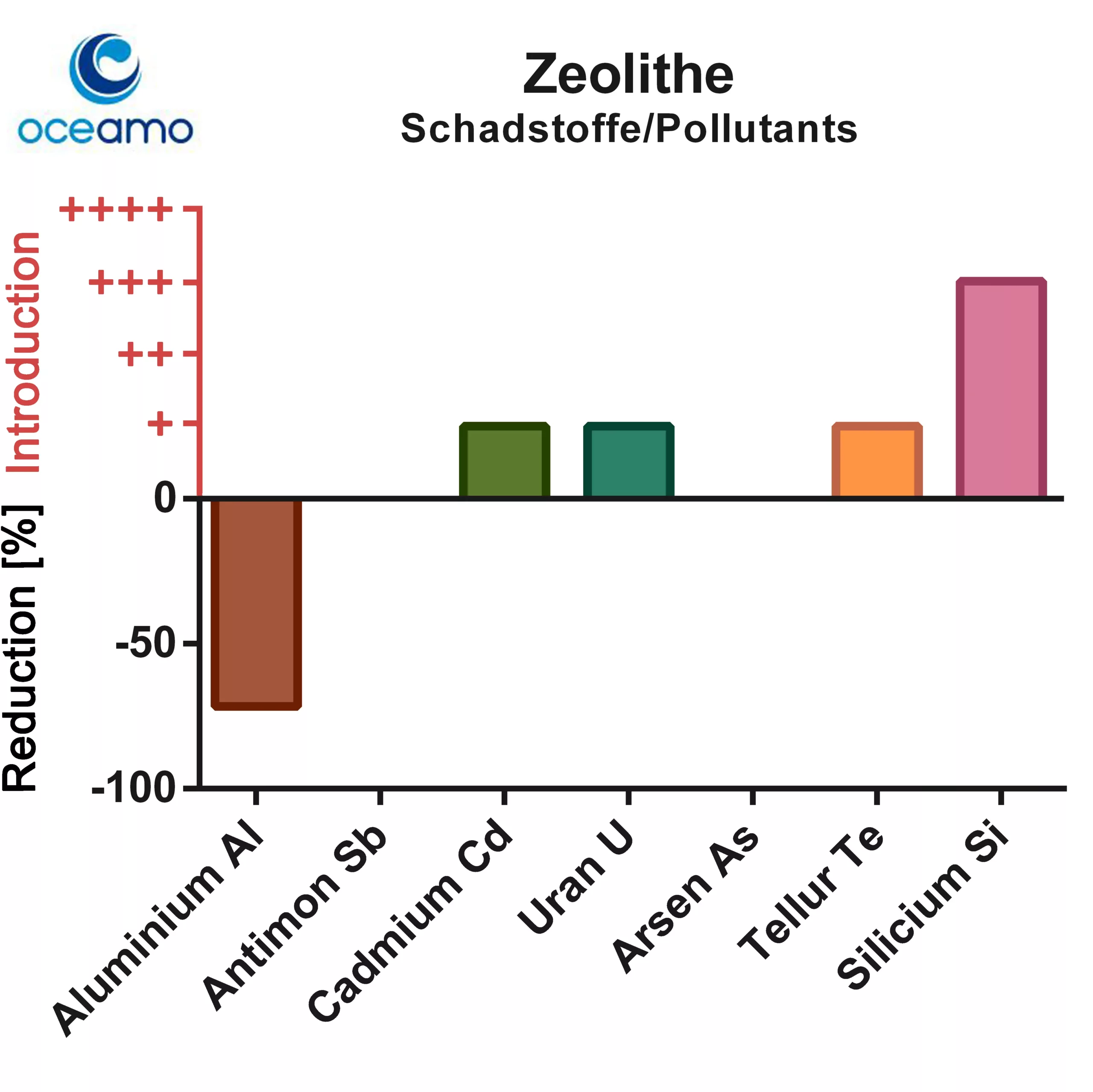
In the experimental setup, zeolite was able to bind aluminum significantly, a property that zeolites had not previously been said to have in the aquarium environment. This is particularly surprising since zeolites themselves contain aluminum.
The investigated zeolite released significant amounts of silicon into the sea water – a small release of cadmium, uranium and tellurium also occurred (toxic concentrations are not reached with typical amounts used in reef aquariums!)
A small release of the toxic heavy metal thallium could also be observed (not shown in the diagram).
The concentrations of antimony and arsenic were not affected by the zeolite.

The activated carbon investigated led to the entry of pollutants, with arsenic and silicon being particularly noteworthy.
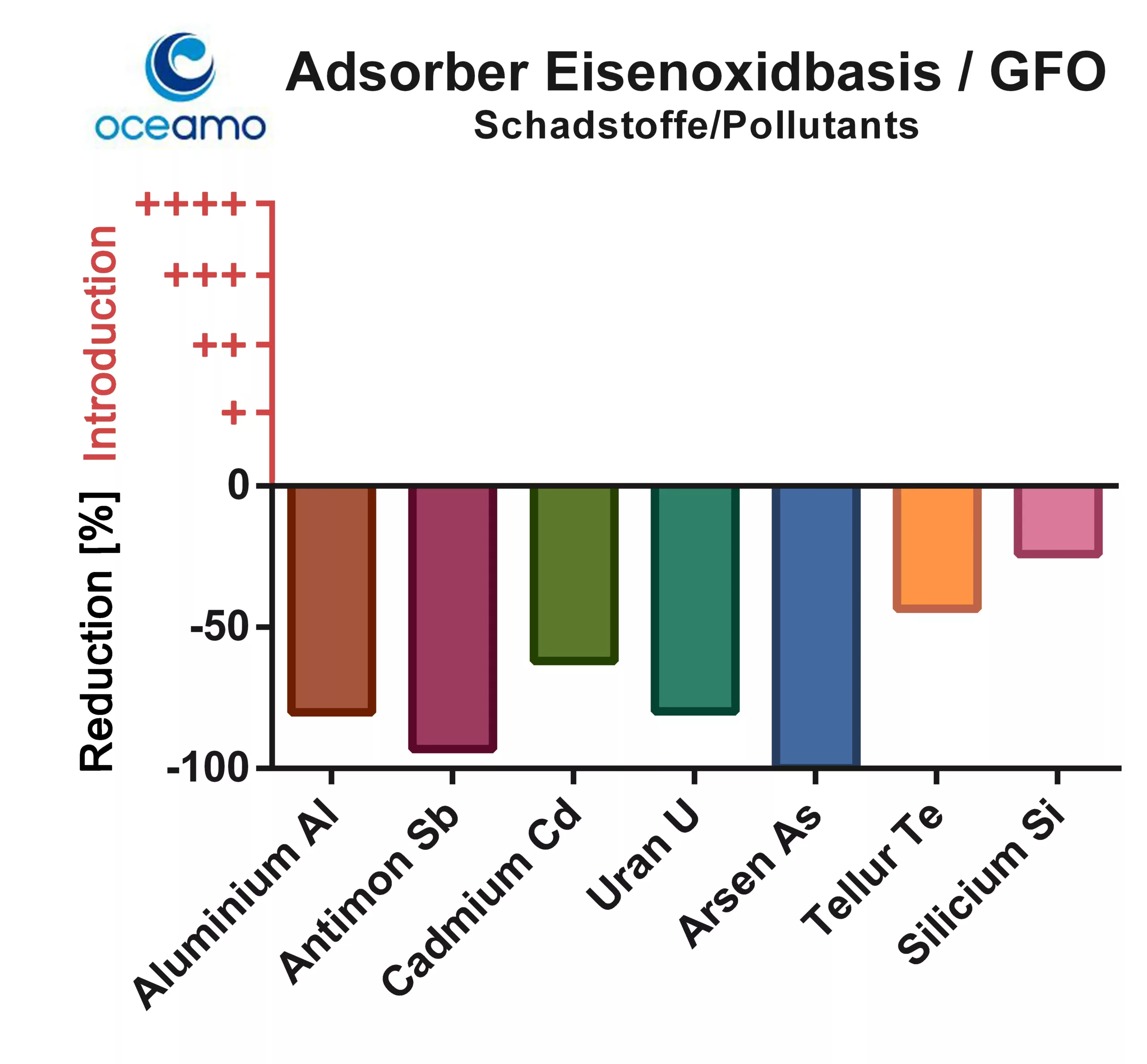
The iron-based adsorber did not lead to any measurable introduction of pollutants, but there was a lowering effect on almost all tested elements.
Particularly noteworthy is the strong binding of the frequently problematic substances aluminium, antimony, arsenic and cadmium. Significantly less was the influence on tellurium and silicon.
For us, interesting (and also unexpected) aspects emerged from this small study, which will also influence the interpretation of our seawater analyses.
The shown data makes it clear that the use of adsorbers should always be considered carefully and should never be taken lightly.
If you have any questions, please contact us!
You can support our work by buying our products (e.g. the ICP-MS analysis for saltwater aquariums), sand by liking us on Facebook and Instagram!

